Clinical outcomes cause by respiratory syncytial virus infection during the first two years of life in patients of a kangaroo program who received complete prophylaxis with palivizumab. Retrospective cohort study
Main Article Content
Abstract
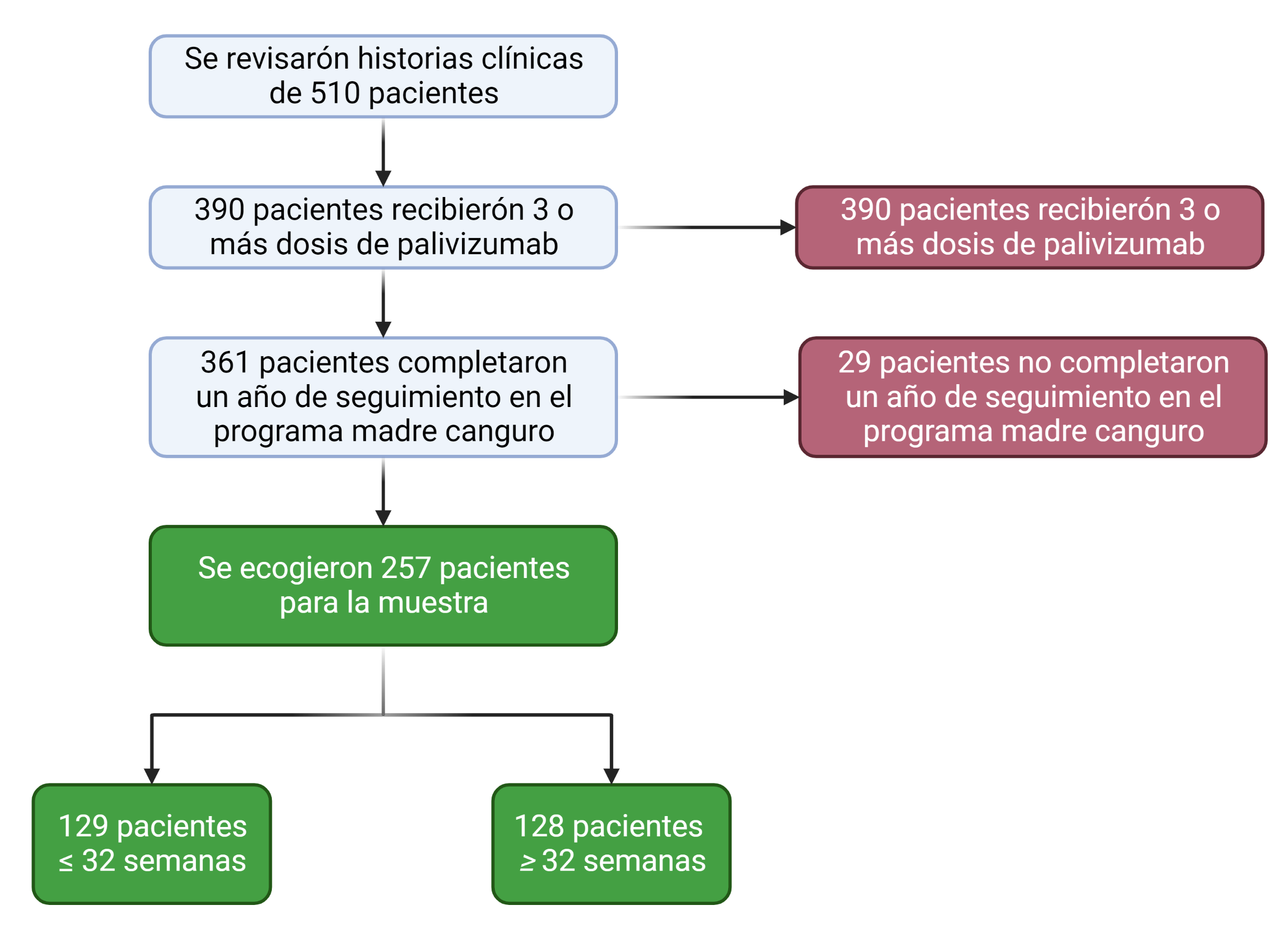
Objective: To estimate the incidence of hospitalizations due to acute lower respiratory tract infection caused by a respiratory syncytial virus during the first two years of life in patients who attended the kangaroo program at Hospital Universitario Clínica San Rafael and who received complete prophylaxis with palivizumab. Methods: An observational retrospective cohort study was conducted. Patients who received total doses of palivizumab during the study period between January 2014 and December 2019 were included. Sociodemographic and clinical variables were evaluated, and descriptive and multivariate statistical techniques were applied to analyze the information. Results: 510 patients were reviewed, and 257 subjects were selected; 128 for the cohort greater than 32 weeks of gestation and 129 for the cohort equal to or less than 32 weeks. Hospitalization due to respiratory syncytial virus occurred in 8.9% of cases. The risk of hospitalization was higher in patients with a gestational age of fewer than 32 weeks, RR: 1.65, 95% CI [1.28 – 2.12], as was hospitalization in the intensive care unit RR: 1.65, 95% CI [1.24 – 2.19] and secondary complications RR: 1.61, 95% CI [1.22 – 2.13]. There were no mortality events. Conclusion: RSV hospitalization is higher at gestational ages less than 32 weeks, and there is no difference according to the clinical variables explored in the study.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Creative Commons
License Attribution-NonCommercial-ShareAlike 4.0 International (CC BY-NC-SA 4.0)
You are free to:
Share - copy and redistribute the material in any medium or format.
Adapt - remix, transform, and build upon the material The licensor cannot revoke these freedoms as long as you follow the license terms.
• Attribution — You must give appropriate credit, provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner, but not in any way that suggests the licensor endorses you or your use.
• NonCommercial — You may not use the material for commercial purposes.
• ShareAlike — If you remix, transform, or build upon the material, you must distribute your contributions under the same license as the original.
• No additional restrictions — You may not apply legal terms or technological measures that legally restrict others from doing anything the license permits.
References
Troeger C, Blacker B, Khalil IA, Rao PC, Cao J, Zimsen SRM, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. noviembre de 2018;18(11):1191-210.
Bauer G, Bossi L, Santoalla M, Rodríguez S, Fariña D, Speranza A. Impacto de un programa de prevención de infecciones respiratorias en lactantes prematuros de alto riesgo: estudio prospectivo y multicéntrico. Arch Argent Pediatr. 2009;107(2):111-8.
Beltran M, Osorio LK, Duran MA, Barbosa J, Ospitia E, Pelaez D. Informe epidemiológico. Virus Sincitial Respiratorio (VST) en menores de 5 años, Colombia, 2012-2016. Instituto Nacional de Salud; 2016.
Instituto Nacional de Salud. Boletín Epidemiológico Semanal. Semana epidemiológica 21. 2019. DOI: https://doi.org/10.33610/23576189.2019.2
Wegzyn C, Toh LK, Notario G, Biguenet S, Unnebrink K, Park C, et al. Safety and Effectiveness of Palivizumab in Children at High Risk of Serious Disease Due to Respiratory Syncytial Virus Infection: A Systematic Review. Infect Dis Ther. diciembre de 2014;3(2):133-58. DOI: https://doi.org/10.1007/s40121-014-0046-6
Feltes TF, Cabalka AK, Meissner HC, Piazza FM, Carlin DA, Top FH, et al. Palivizumab prophylaxis reduces hospitalization due to respiratory syncytial virus in young children with hemodynamically significant congenital heart disease. J Pediatr. octubre de 2003;143(4):532-40. DOI: https://doi.org/10.1067/S0022-3476(03)00454-2
Moore HC, de Klerk N, Richmond PC, Fathima P, Xu R, Keil AD, et al. Effectiveness of Palivizumab against Respiratory Syncytial Virus: Cohort and Case Series Analysis. J Pediatr. noviembre de 2019;214:121-127.e1. DOI: https://doi.org/10.1016/j.jpeds.2019.06.058
The IMpact-RSV Study Group. Palivizumab, a Humanized Respiratory Syncytial Virus Monoclonal Antibody, Reduces Hospitalization From Respiratory Syncytial Virus Infection in High-risk Infants. PEDIATRICS 1998;102(3):531-7. DOI: https://doi.org/10.1542/peds.102.3.531
Castillo LM, Bugarin G, Arias JC, Barajas Rangel JI, Serra ME, Vain N. One-year observational study of palivizumab prophylaxis on infants at risk for respiratory syncytial virus infection in Latin America. J Pediatr (Rio J). septiembre de 2017;93(5):467-74. DOI: https://doi.org/10.1016/j.jped.2016.11.006
Instituto de Evaluación tecnológica en Salud. Efectividad de palivizumab para la reducción de riesgo de infección respiratoria por virus sincitial respiratorio en el recién nacido prematuro. La institución. Bogota: 2013.
Nuijten MJ, Wittenberg W. Cost effectiveness of palivizumab in Spain: an analysis using observational data. Eur J Health Econ 2010;11(1):105-15. DOI: https://doi.org/10.1007/s10198-009-0206-x
Carbonell-Estrany X, Lázaro y de Mercado P. Health economics and RSV. Paediatr Respir Rev 2009; 10 Suppl 1:12-3. DOI: https://doi.org/10.1016/S1526-0542(09)70006-5
Reeve CA, Whitehall JS, Buettner PG, Norton R, Reeve DM, Francis F. Cost-effectiveness of respiratory syncytial virus prophylaxis with palivizumab. J Paediatr Child Health 2006; 42(5):253-8. DOI: https://doi.org/10.1111/j.1440-1754.2006.00850.x
Romero JR. Palivizumab prophylaxis of respiratory syncytial virus disease from 1998 to 2002: results from four years of palivizumab usage. Pediatr Infect Dis J 2003 Feb;22(2 Suppl):S46-54. DOI: https://doi.org/10.1097/01.inf.0000053885.34703.84
Stewart DL, Ryan KJ, Seare JG, Pinsky B, Becker L, Frogel M. Association of RSV-related hospitalization and non-compliance with palivizumab among commercially insured infants: a retrospective claims analysis. BMC Infect Dis 2013;13:334. DOI: https://doi.org/10.1186/1471-2334-13-334
Cetinkaya M, Oral TK, Karatekin S, Cebeci B, Babayigit A, Yesil Y. Efficacy of palivizumab prophylaxis on the frequency of RSV-associated lower respiratory tract infections in preterm infants: determination of the ideal target population for prophylaxis. Eur J Clin Microbiol Infect Dis 2017; 36(9):1629-1634. DOI: https://doi.org/10.1007/s10096-017-2976-x
Luna MS, Manzoni P, Paes B, Baraldi E, Cossey V, Kugelman A, Chawla R, Dotta A, Rodríguez Fernández R, Resch B, Carbonell-Estrany X. Expert consensus on palivizumab use for respiratory syncytial virus in developed countries. Paediatr Respir Rev. 2020;33:35-44. DOI: https://doi.org/10.1016/j.prrv.2018.12.001
Azzari C, Baraldi E, Bonanni P, Bozzola E, Coscia A, Lanari M, Manzoni P, Mazzone T, Sandri F, Checcucci Lisi G, Parisi S, Piacentini G, Mosca F. Epidemiology and prevention of respiratory syncytial virus infections in children in Italy. Ital J Pediatr. 2021;47(1):198. DOI: https://doi.org/10.1186/s13052-021-01148-8
Paes B. Respiratory Syncytial Virus in Otherwise Healthy Prematurely Born Infants: A Forgotten Majority. Am J Perinatol 2018;35(6): 541-544. DOI: https://doi.org/10.1055/s-0038-1637762
Lin YJ, Chung CH, Chi H, Lin CH. Six-monthly palivizumab prophylaxis effectively reduced RSV-associated hospitalization rates of preterm infants in a subtropical area: a population-based cohort study. Pediatr Res 2019;86(5):628-634. DOI: https://doi.org/10.1038/s41390-019-0492-7
Yeo KT, Yung CF, Khoo PC, Saffari SE, Peng Sng JS, How MS, Quek BH. Effectiveness of Palivizumab Against Respiratory Syncytial Virus Hospitalization Among Preterm Infants in a Setting With Year-Round Circulation. J Infect Dis 2021;224(2):279-287. DOI: https://doi.org/10.1093/infdis/jiaa749